GEPHART lAB
GEPHART lAB
GEPHART LAB
STANFORD NEUROSURGERY
OUR VISION
Our laboratory accelerates translational brain tumor research, combining innovative techniques in genetics and cancer biology with a unique insight into the pressing clinical questions facing patients. We use novel genetic sequencing methods and modeling to understand how cancer grows in the brain, inadvertently supported by native brain cells. We focus on translational therapeutic targets identified from and validated with primary human tissue and cerebrospinal fluid whenever possible, as this most reliably reflects the human disease. These findings have led to novel diagnostic tools and clinical trials for patients with malignant brain tumors. Our laboratory is a dynamic and collaborative working environment, benefitting from the supportive research environment at Stanford. Our laboratory space bridges the Stanford core campus and the medical facilities, emblematic of the translational aspects of our work.
SELECTED PUBLICATIONS

A Novel Brain-Permeant Chemotherapeutic Agent for the Treatment
of Brain Metastasis
in Triple-Negative Breast Cancer
Mol Cancer Ther. 2021 Nov;20(11):2110-2116
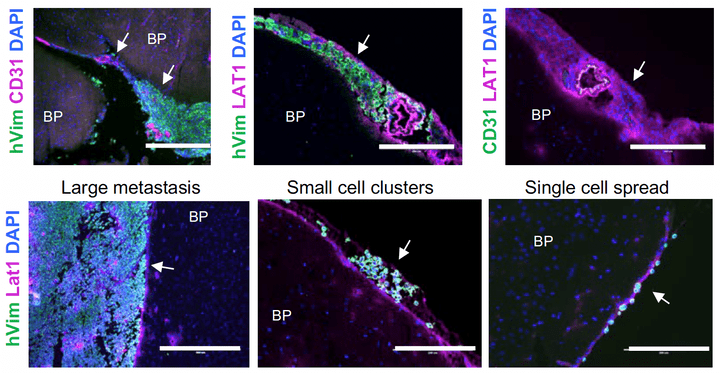
Development of metastases to central nervous system (CNS) is an increasing clinical issue following the diagnosis of advanced breast cancer. The propensity to metastasize to CNS varies by breast cancer subtype. Of the four breast cancer subtypes, triple-negative breast cancers (TNBC) have the highest rates of both parenchymal brain metastasis and leptomeningeal metastasis (LM). LM is rapidly fatal due to poor detection and limited therapeutic options. Therapy of TNBC brain metastasis and LM is challenged by multifocal brain metastasis and diffuse spread of LM, and must balance brain penetration, tumor cytotoxicity, and the avoidance of neurotoxicity. Thus, there is an urgent need for novel therapeutic options in TNBCs CNS metastasis. QBS10072S is a novel chemotherapeutic that leverages TNBC-specific defects in DNA repair and LAT1 (L-amino acid transporter type 1)-dependent transport into the brain.
Read more at:

Comprehensive RNA analysis of CSF reveals a role for CEACAM6 in lung cancer leptomeningeal metastases
NPJ Precis Oncol. 2021 Oct 8;5(1):90
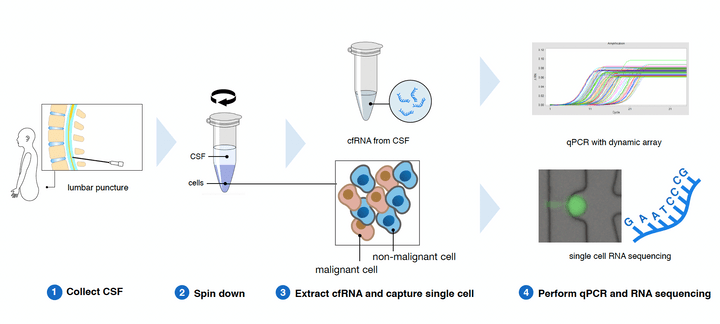
Non-small cell lung cancer (NSCLC) metastatic to the brain leptomeninges is rapidly fatal, cannot be biopsied, and cancer cells in the cerebrospinal fluid (CSF) are few; therefore, available tissue samples to develop effective treatments are severely limited. This study aimed to converge single-cell RNA-seq and cell-free RNA (cfRNA) analyses to both diagnose NSCLC leptomeningeal metastases (LM), and to use gene expression profiles to understand progression mechanisms of NSCLC in the brain leptomeninges.
Read more at:

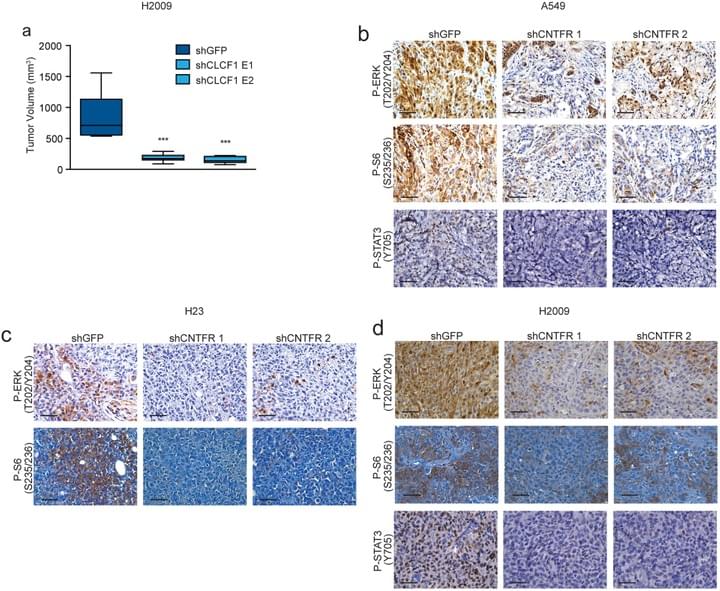
Antitumor activity of an engineered decoy receptor targeting CLCF1-CNTFR signaling in lung adenocarcinoma
Nature Medicine. 2019 Nov;25(11):1783-1795.
Proinflammatory cytokines in the tumor microenvironment can promote tumor growth, yet their value as therapeutic targets remains underexploited. We validated the functional significance of the cardiotrophin-like cytokine factor 1 (CLCF1)-ciliary neurotrophic factor receptor (CNTFR) signaling axis in lung adenocarcinoma (LUAD) and generated a high-affinity soluble receptor (eCNTFR-Fc) that sequesters CLCF1, thereby inhibiting its oncogenic effects. eCNTFR-Fc inhibits tumor growth in multiple xenograft models and in an autochthonous, highly aggressive genetically engineered mouse model of LUAD, driven by activation of oncogenic Kras and loss of Trp53.
Read more at:

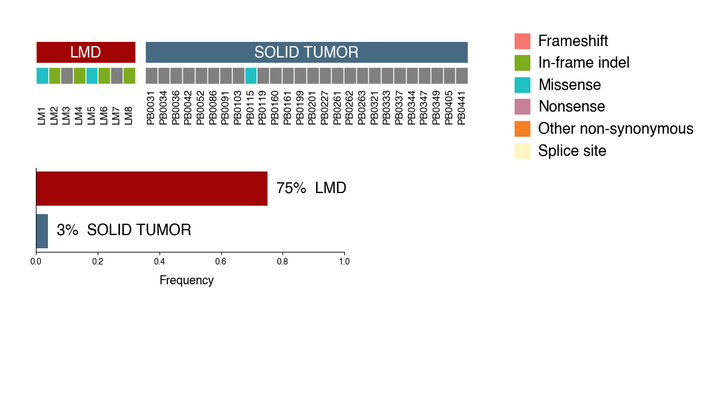
Recurrently Mutated Genes Differ between Leptomeningeal and Solid Lung Cancer Brain Metastases.
J Thorac Oncology 2018 Jul;13(7):1022-1027.
When compared with solid brain metastases from NSCLC, leptomeningeal disease (LMD) has unique growth patterns and is rapidly fatal. Patients with LMD do not undergo surgical resection, limiting the tissue available for scientific research. In this study we performed whole exome sequencing on eight samples of LMD to identify somatic mutations and compared the results with those for 26 solid brain metastases.
Read more at:

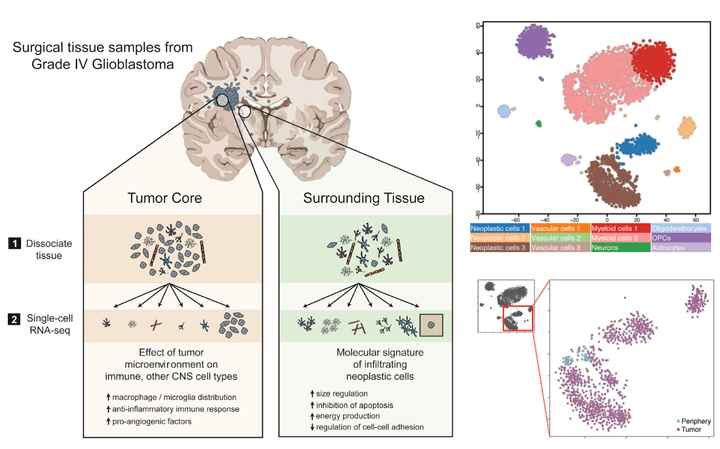
Single-Cell RNA-Seq Analysis of Infiltrating Neoplastic Cells at the Migrating Front of Human Glioblastoma
Cell Reports. 2017 Oct 31; 21(5):1399-410.
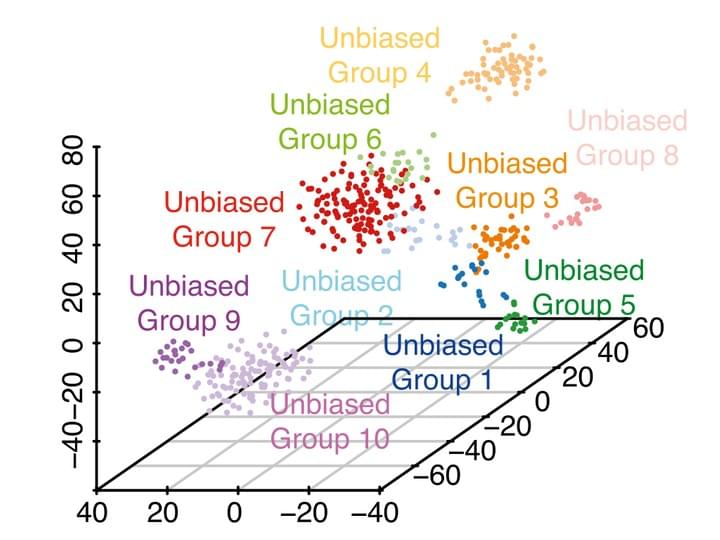
Glioblastoma (GBM) is the most common primary brain cancer in adults and is notoriously difficult to treat because of its diffuse nature. We performed single-cell RNA sequencing (RNA-seq) on 3,589 cells in a cohort of four patients. We obtained cells from the tumor core as well as surrounding peripheral tissue. Our analysis revealed cellular variation in the tumor's genome and transcriptome. We were also able to identify infiltrating neoplastic cells in regions peripheral to the core lesions. Despite the existence of significant heterogeneity among neoplastic cells, we found that infiltrating GBM cells share a consistent gene signature between patients, suggesting a common mechanism of infiltration. Additionally, in investigating the immunological response to the tumors, we found transcriptionally distinct myeloid cell populations residing in the tumor core and the surrounding peritumoral space. Our data provide a detailed dissection of GBM cell types, revealing an abundance of information about tumor formation and migration.
Explore data at:
Read more at:
http://www.cell.com/cell-reports/fulltext/S2211-1247(17)31462-6

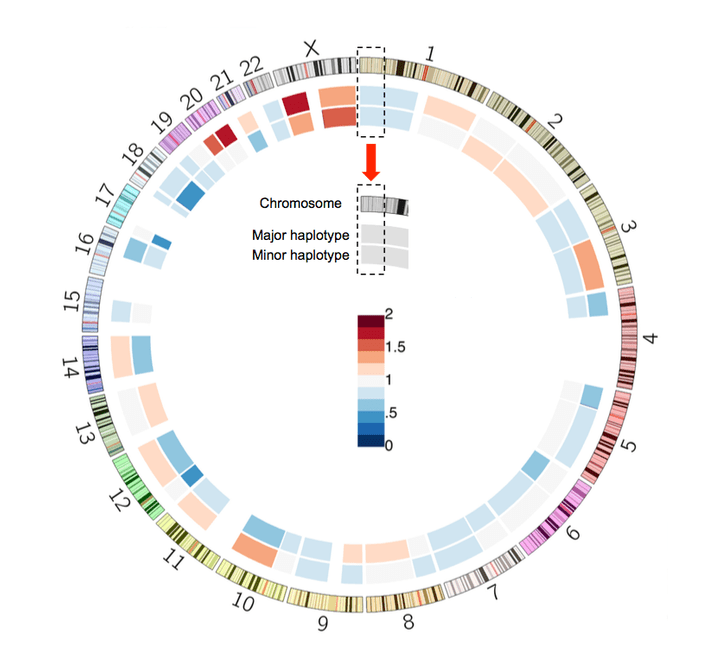
Chromosome-scale mega-haplotypes enable digital karyotyping of cancer aneuploidy.
Nucleic Acids Research. 2017 Aug 16.
Genomic instability is a frequently occurring feature of cancer that involves large-scale structural alterations. These somatic changes in chromosome structure include duplication of entire chromosome arms and aneuploidy where chromosomes are duplicated beyond normal diploid content. However, the accurate determination of aneuploidy events in cancer genomes is a challenge. Recent advances in sequencing technology allow the characterization of haplotypes that extend megabases along the human genome using high molecular weight (HMW) DNA. For this study, we employed a library preparation method in which sequence reads have barcodes linked to single HMW DNA molecules. Barcode-linked reads are used to generate extended haplotypes on the order of megabases. We developed a method that leverages haplotypes to identify chromosomal segmental alterations in cancer and uses this information to join haplotypes together, thus extending the range of phased variants. With this approach, we identified mega-haplotypes that encompass entire chromosome arms. We characterized the chromosomal arm changes and aneuploidy events in a manner that offers similar information as a traditional karyotype but with the benefit of DNA sequence resolution. We applied this approach to characterize aneuploidy and chromosomal alterations from a series of primary colorectal cancers.
Read more at:

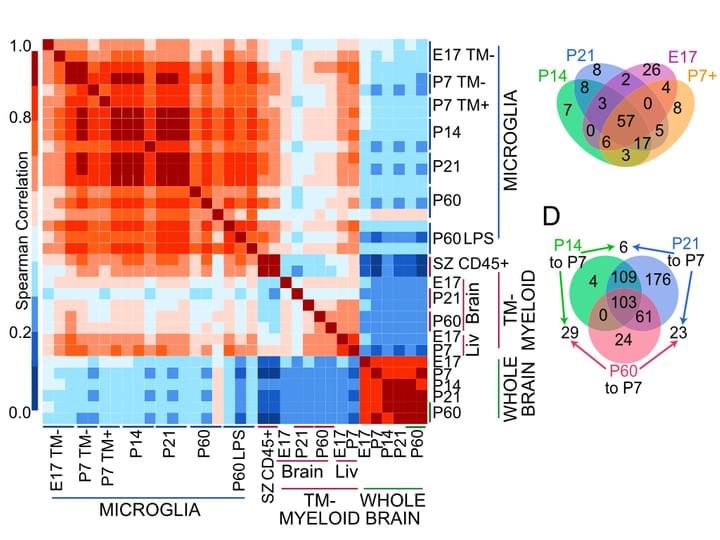
New tools for studying microglia in the mouse and human CNS.
Proc Natl Acad Sci U S A. 2016 Mar 22;113(12)
Here, we identify transmembrane protein 119 (Tmem119), a cell-surface protein of unknown function, as a highly expressed microglia-specific marker in both mouse and human. We developed monoclonal antibodies to its intracellular and extracellular domains that enable the immunostaining and isolation of microglia. Using our antibodies, we provide, to our knowledge, the first RNAseq profiles of highly pure mouse microglia during development and after an immune challenge.
Read more at:

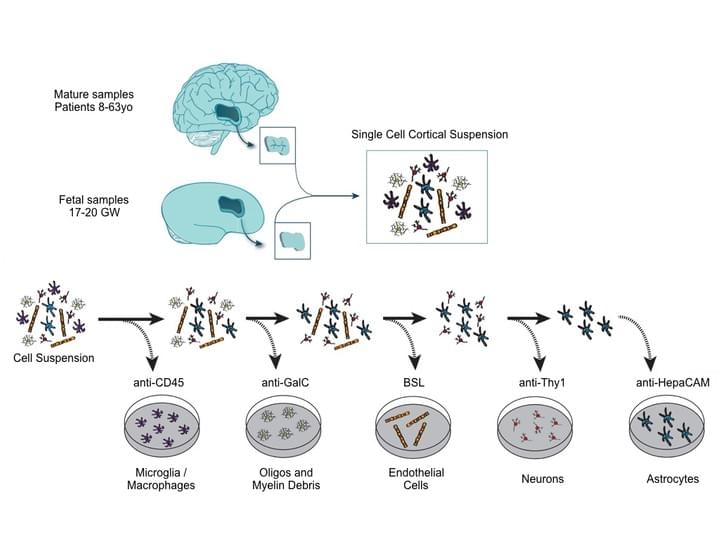
Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse.
Neuron | January 2016 | Volume 89, Issue 1, 6 | Pages 37–53
The functional and molecular similarities and distinctions between human and murine astrocytes are poorly understood. Here, we report the development of an immunopanning method to acutely purify astrocytes from fetal, juvenile, and adult human brains and to maintain these cells in serum-free cultures.
Read more at:

A survey of human brain transcriptome diversity at the single cell level.
PNAS | June 9, 2015 | vol. 112 | no. 23 | 7285–7290
The human brain is a tissue of vast complexity in terms of the cell types it comprises. Conventional approaches to classifying cell types in the human brain at single cell resolution have been limited to exploring relatively few markers and therefore have provided a limited molecular characterization of any given cell type. We used single cell RNA sequencing on 466 cells to capture the cellular complexity of the adult and fetal human brain at a whole transcriptome level.
Read more at:

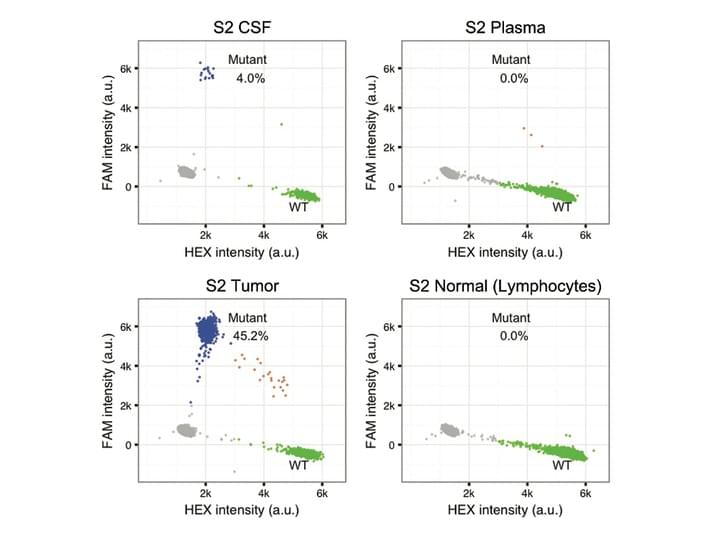
Brain tumor mutations detected in cerebral spinal fluid.
Clin Chem. 2015 Mar;61(3):514-22.
Detecting tumor-derived cell-free DNA (cfDNA) in the blood of brain tumor patients is challenging, presumably owing to the blood-brain barrier. Cerebral spinal fluid (CSF) may serve as an alternative "liquid biopsy" of brain tumors by enabling measurement of circulating DNA within CSF to characterize tumor-specific mutations. Many aspects about the characteristics and detectability of tumor mutations in CSF remain undetermined. We detected tumor mutations in CSF samples from 6 of 7 patients with solid brain tumors. The concentration of the tumor mutant alleles varied widely between patients, from <5 to nearly 3000 copies/mL CSF. We identified 7 somatic mutations from the CSF of a patient with leptomeningeal disease by use of cancer panel sequencing, and the result was concordant with genetic testing on the primary tumor biopsy. Tumor mutations were detectable in cfDNA from the CSF of patients with different primary and metastatic brain tumors. We designed 2 strategies to characterize tumor mutations in CSF for potential clinical diagnosis: the targeted detection of known driver mutations to monitor brain metastasis and the global characterization of genomic aberrations to direct personalized cancer care.
Read more at:
lAB MEMBERS
Our team combines experience with passion, creativity, and dedication.

Melanie Hayden Gephart
Principal Investigator
Dr. Hayden Gephart did her residency and post doctoral training at Stanford University in neurosurgery and cancer biology. She joined as faculty in 2014 and is a brain tumor neurosurgeon.

Sophia Chernikova
Senior Researcher
Sophia is a cancer biologist with an expertise in DNA repair, epigenetics, genomic instability and radiation oncology. Her present interests are in brain tumors with a specific focus on brain metastases from triple negative breast cancer. She enjoys data mining, hiking and biking.

Shruti Jain
Post-doctoral Researcher
Shruti has obtained her Ph.D. in Neuroscience from Kent State University, Ohio in 2017. She has extensive experience in developmental neurobiology and her Ph.D. work focused on local translation of mRNAs during brain development in Down syndrome. She enjoys traveling, painting, and Zumba.

Maxine Umeh - Garcia
Post-doctoral Researcher
Maxine obtained her PhD in Biochemistry, Molecular, Cell and Developmental Biology with an emphasis in Translational Research from UC Davis. She has an extensive background in cancer biology and an interest in triple negative breast cancer, which has a higher incidence and death rate in African-American women. She enjoys volleyball, piano playing, analyzing data, and making pretty graphs.

Samuel Wong
Post-doctoral Researcher
Sam obtained his PhD in Cellular and Molecular Medicine from Johns Hopkins University, Baltimore in 2020. He has experience in developmental neurobiology and enjoys reading and hiking in his free time.

Giuseppe Barisano
Post-doctoral Researcher
Giuseppe earned his M.D. at San Raffaele University and his Ph.D in Neuroscience at University of Southern California. He has an extensive backgfound in neuroimaging research and single-cell omics approaches. He is interested in identifying novel neuroimaging and molecular biomarkers of brain tumors. He enjoys cycling, running, hiking and being in nature.

Minkyung Kang
Post-doctoral Researcher
Min earned her PhD in Medical Sciences from University of South Florida, where she developed in-depth expertise in the blood-brain barrier. Her current research is centered on investigating the blood-tumor barrier as an interface of brain metastases and a target for cancer therapy delivery. Outside the laboratory, she enjoys playing the violin and exploring new places.

Crystal Wang
Clinical Fellow
Crystal is a Stanford pediatric hematology/oncology fellow interested in translational oncology research. She went to medical school at Johns Hopkins University School of Medicine and completed her pediatrics residency training at St. Christopher's Hospital for Children in Philadelphia. She enjoys cooking and going to the beach.

Georgiana Burnside
Medical Student
Georgiana is a recent graduate of Stanford University where she studied neurobiology. She is interested in clinical and translational research approaches to spinal cord and brain injury medicine and plans to pursue medical school in the next few years. In her free time, she enjoys watching docuseries, camping, and spending time with family.

Vaibhavi Bhaviesh Shah
Medical Student
Vaibhavi is a medical student at Stanford interested in neurosurgery. She completed her undergraduate in Biological Engineering and in Science, Technology, and Society at the Massachusetts Institute of Technology (MIT) in 2021. She enjoys playing tennis, running, and listening to music in her free time.

Charlotte Weixel
Medical Student
Charlotte is a medical studentat Stanford interested in neurosurgery. She graduated from Washington University in St. Louis in 2023 where she studied Biomedical Engineering and researched applications of focused ultrasound in the brain. In her free time, she enjoys running, ceramics, and trying new coffee shops.

George Rafael Nageeb
Medical Student
George is a medical student at Stanford interested in neurosurgery. He graduated from Harvard University in 2022 where
he studied Biomedical Engineering and Comparative Studies of Religion. He was
also a member of Harvard's Varsity Soccer Team. His previous research focused on tissue engineering and mechano-biological mechanisms of disease. In his free time, he enjoys woodworking, reading, and watching reality TV.
Claudia Leonard
Ph.D. Student
Claudia is a Ph.D Student in the Cancer Biology program at the Stanford School of Medicine. She obtained her Bachelor’s degree in Biology with a specialization in Cell Biology, Molecular Biology, and Genetics from Boston University. She also earned her Master’s degree in Biology from Harvard University. She is
interested in identifying biomarkers of brain metastasis at the proteomic level
in different subsets of patient populations and studying health disparities within clinical trials. Outside of lab, she enjoys dancing, reading, listening
to music, and spending time with friends and family.
Brandon Carlson-Clarke
Clinical Research Coordinator
Brandon is working as a Clinical Research Coordinator with the Cancer Clinical Trials Office and the Brain Tumor Center and hopes to attend medical school with interest in neurosurgery. He completed his undergraduate degree in neurobiology and behavior at Cornell University. He enjoys skiing, camping, and cooking.

Monica Reddy
Clinical Research Coordinator
Monica is a Clinical Research Coordinator in the Stanford Cancer Institute Clinical Trials Office (SCI-CTO). As part of SCI-CTO, she supports the activities related to the clinical trials within the Neurosurgery and Neuro-Oncology areas. She completed her Bachelor of Science
degree at the Medical College of Georgia and later pursued and completed her
MBA at San Jose State University. In her spare time, she enjoys hiking, traveling, and spending time with family and friends.
Sarah Tedjakusuma
Clinical Research
Coordinator Associate
Sarah is a Clinical Research Coordinator
in the Stanford Cancer Institute Clinical Trials Office. Shesupports and facilitates the daily clinical trial activities of Stanford’s Neurosurgery Department’s clinical trials. She completed her undergraduate degree in Molecular, Cell, and Developmental Biology at UC Santa Cruz. In her free time, she enjoys practicing yoga, cooking, and trying new restaurants.

Shreya Kumar
Clinical Research
Coordinator Associate
Shreya is a recent graduate of UT Austin with a Bachelor of Science in Neuroscience and a Minor in American Sign Language.She hopes to attend medical school and is interested in Neurosurgery. She is passionate about finding solutions to making healthcare more equitable, especially for the deaf community. In her free time, she enjoys singing, hiking, and crafty projects.

Sahara Shanti Rout
Clinical Research Coordinator
Sahara is a Clinical Research Coordinator at the Stanford CancerInstitute Clinical Trials Office (SCI-CTO). Sahara supports and facilitates the activities related to clinical trials within Neurosurgery and Neuro-Oncology. She completed her Bachelor of Science in Neuroscience with a double minor in Global Health and Psychology & Master of Arts in Global Health at UC San Diego. In her free time, Sahara enjoys swimming, hiking, baking, and trying new restaurants.

Zachary Jun Xiang Yu
Undergraduate Researcher
Zachary is an undergraduate at Stanford. He intends to study both Psychology and Biology, with a focus in cognitive neuroscience and medicine. In his free time, Zach enjoys cooking, playing ultimate frisbee and volleyball.

Pablo Nunez
Undergraduate Researcher
Pablo is an undergraduate at Stanford studying bioengineering. He hopes to attend medical school and is interested in clinical and translational research pertaining to cancer. Outside of the lab, he plays on the Stanford Club Soccer team and volunteers as a Spanish interpreter for the Cardinal Free Clinics. He enjoys running, playing soccer, and spending time outdoors.

Debbie Bong
Undergraduate Researcher
Debbie is an undergraduate at Stanford planning to study Symbolic Systems with a concentration in neuroscience. She is interested in disparities of medicine. In her free time, she enjoys baking, working out, and trying new restaurants.

Sofia Marie Tosoni
Undergraduate Researcher
Sofia is an undergraduate at Stanford majoring in Bioengineering with a Notation in Science Communication. She's passionate about oncology and targeted cancer therapies and plan to attend medical school in the future. In her free time, Sofia loves to run and mentor young girls in STEM. Sofia is also currently training to become a student guide at the Cantor Arts Center.

Ayushi Mohanty
Undergraduate Researcher
Ayushi is an undergraduate at Stanford planning to major in Bioengineering. She is interested in learning more about the intersection of neuroscience and technology. Outside of the lab, Ayushi is an executive member of the Stanford Undergraduate Research Association and Stanford Undergraduate Neuroscience Society. In her free time, she enjoys dancing, solving puzzles, and spending time with family.

Christine Xie
Undergraduate Researcher
Christine is an undergraduate at Stanford studying Biomedical Computation. She is interested in neuro-oncology and plans to attend medical school. In her free time, Christine enjoys working out with friends, testing new baking recipes, and listening to music.

Thy Trinh
Life Science Research Professional 1
Thy obtained Bachelor of Science in Biochemistry at Denison University before joining the lab. She is interested in healthcare and biomedical research, particularly in topics about human physiology and diseases. Thy enjoys cooking, biking, and singing in her free time.

Rukayat Taiwo
Neurosurgery Resident
Rukayat is a Stanford Neurosurgery Resident. She completed an MD/MA at Washington University in Saint Louis where she studied epigenetic factors important in the tumorigenicity of glioblastoma stem cells. She is currently interested in studying the brain tumor microenvironment to improve our understanding and treatment of brain cancers. In her free time, Rukayat enjoys eating her way around the world and exploring new cultures.

Daniel Herrick
Neurosurgery Resident
Dan Herrick is a Stanford Neurosurgery resident and recently completed the Stanford Biodesign Innovation Fellowship. He earned his M.D. and Ph.D. degrees at Tufts University School of Medicine where his Ph.D. research identified novel molecular mechanisms governing adult neurogenesis after injury. He is interested in translational research focused on identifying biomarkers of neurotrauma.

Meaghan Roy-O'Reilly
Neurology Resident
Meaghan is a current adult Neurology resident andfuture Stanford Neuro-oncology fellow. She obtained her MD/PhD at UT Houston in
Neuroscience and Immunology. She enjoys playing the drums, swimming and her dog Guinness.
Julie Jin Huang
Research Administrator
Julie obtained aM.S.Accountancy and has worked in Accounting/Finance field before. Now, she is supporting Dr. Hayden and the lab with research & administration. She enjoys playing pickleball; listening to audio books and travel.
Neuro-oncology research, it's personal
Neurosurgery isn't just my career and practice, it is a personal struggle to help improve the treatment for patients with brain tumors. These diseases have taken the lives of many of my friends and family, and affects the lives of my patients every day. Just a handful of their photos are listed here. We are committed to improving the care of patients with brain tumors and understanding the underlying mechanisms of disease progression, motivated by a personal understanding of the disease. I work in the operating room employing the maximal treatment for patients in my clinic today. The lab looks to develop new treatments and diagnostic techniques for the future patients who will be in the clinic tomorrow.

Robert Bean (1966 - 2000)
Robert Bean, Dr. Hayden's uncle, passed away at the age of 37 from Glioblastoma.

Paul Kalanithi (1977 - 2015)
Paul, Dr. Hayden's co-resident, was diagnosed with metastatic lung cancer during his neurosurgery residency. He passed away at the age of 37.
ALUMNI
Wenying Pan
Position in the Lab (2013 - 2016): Graduate Student
Subsequent position Scientist, Grail Inc.
Linya You
Position in the Lab (2015 - 2016): Post doctorate
Subsequent position: Medical student, Shanghai Medical College of Fudan University
Yingmei Li
Position in the Lab (2014 - 2018): Post doctorate
Subsequent position: Scientist, BindeBio and Chinese Academy of Science.
Ian Connolly
Position in the Lab (2014 - 2019) : Medical Student
Subsequent position: Neurosurgery Resident Physician, Massachusetts General Hospital
Jiaojiao Deng
Position in the Lab (2018 - 2019): Medical Student
Subsequent position: Neurosurgery student, Huashan Hospital, Fudan University.
Eli Johnson
Position in the Lab (2015 - 2020): Medical Student
Subsequent position: Neurosurgery Resident, Duke University.
Dina Polyak
Position in the Lab (2017 - 2020): Post doctorate
Subsequent position: Scientist, Arsenal Bio.
Lina Khav Khoeur
Position in the Lab (2015 - 2018): Undergraduate
Subsequent position: Medical Student, UCSF
Yuelong Wang
Position in the Lab (2019 - 2020): Visiting Post doctorate
Subsequent position: Assistant Researcher, and Postdoctoral fellow Sichuan University, Chengdu, China
Bina Kakusa
Position in the Lab (2019 - 2021): Medical Student
Subsequent position: Neurosurgery Resident, Stanford
Anna Diaz
Position in the Lab (2021): Summer Intern
Subsequent position: Student, UC Berkeley
Amanda Luu
Position in the Lab (2021): Summer Intern
Subsequent position: Student at UCSD, Majoring in Neuroscience
Hriday P. Bhambhvani
Position in the Lab (2017-2022): Medical Student
Subsequent position: Urology Resident Physician, Cornell University
Steffi Jean Andersen
Position in the Lab (2018-2022): Undergraduate
Subsequent position: MD/PhD Student, Columbia University
Bryanna Godfrey
Position in the Lab (2019-2022): Undergraduate
Subsequent position: MD/PhD student, Columbia University
Saif Ali
Position in the Lab (2022): Summer Research Student
Subsequent position: Student at Stanford University
Juliana Nava
Position in the Lab (2022): Summer Intern
Subsequent position: Student at Cañada College
Adrian Rodrigues
Position in the Lab (2019-2023): Medical Student
Subsequent position: Neurosurgery Resident Physician, Massachusetts General Hospital
Leeza Kopaeva
Position in the Lab (2022-2023): Clinical Research Coordinator
Subsequent position: Clinical Research Coordinator, Stanford Otolaryngology
Kriti Lalwani
Position in the Lab (2023-2024): Clinical Research Coordinator
Subsequent position: MD student, Arizona State University
Megan Lee
Position in the Lab (2023-2024): Visiting medical student researcher
Subsequent position: MD student, The Chinese University of Hong Kong
SUPPORT OUR RESEARCH
A gift to the Gephart Lab will support our research on brain tumors.
If you would like to make a donation for this purpose, please click below or contact:Toba Rainess
Medical Center Development
Phone: (202) 527 3176
Email: Toba.Rainess@stanford.eduInterested in joining our team?
Please submit a CV with a brief description of your research interests and career goals to Melanie Hayden Gephart (mghayden@gmail.com)
Neurosurgical Oncology Instructorship
The Clinical Instructorship is a one-year appointment, July 1 through June 30, with the possibility of a one-year extension for exceptional performance. This position is intended to supplement the core residency training in neurosurgery by providing advanced knowledge and skills of the management of patients with brain tumors, as well as protected time to complete a research project and submit a mentored (e.g., K12, K08) or independent (e.g., R01, U01) federal award. Applicants are encouraged to apply early to allow time for grant submissions prior to the Instructorship. Strong candidates will be senior US neurosurgery residents with a strong track record of academic productivity, and solid plans to transition to an independent neurosurgeon-scientist career. Independent clinical responsibilities will be balanced with 50% protected research time at Stanford University. Additional didactics and conferences are included.
Applications are accepted on a rolling basis. Please send aletter of interest and CV to mghayden@stanford.edu.
CONTACT US
Melanie Hayden Gephart, MD, MAS
Department of Neurosurgery
213 Quarry Rd
4th Fl MC 5958Office (650) 723-8591
mghayden@stanford.eduClinic
Department of Neurosurgery
875 Blake Wilbur Drive, Clinic D
Palo Alto, California 94304
Clinic (650) 497-7777
Stanford Main Hospital (650) 723 4000Office
1201 Welch Rd., MSLS P307
Stanford, CA 94305-5487
Office (650) 723-8591Brain Tumor Center
Clinic Referral/Questions
650-497-7777